Ozone Water Treatment
Bubble Diffusers
Bubble diffusion is the oldest and simplest method for dissolving ozone into water. This is essentially a porous device used for breaking the gas into small bubbles at the bottom of a water column to allow the bubbles to slowly rise to the top of the column and dissolve into water.Pore Size
 The pore size of the diffuser will affect the size of the gas bubbles that are created with the bubble diffuser. Two smaller bubbles will have greater surface area than one bubble of the same gas volume. Greater surface area will achieve improved contact with the gas bubble and water, therefore increasing the rate of mass transfer of ozone into water. It is important when choosing a bubble diffuser to find the smallest pore size possible.
The pore size of the diffuser will affect the size of the gas bubbles that are created with the bubble diffuser. Two smaller bubbles will have greater surface area than one bubble of the same gas volume. Greater surface area will achieve improved contact with the gas bubble and water, therefore increasing the rate of mass transfer of ozone into water. It is important when choosing a bubble diffuser to find the smallest pore size possible.
Water Column Height
The height of the water column that ozone is bubbled into will affect the mass transfer efficiency greatly. The diffuser should be placed at the bottom of the column, this way, the gas bubble must travel the greatest distance within the water column prior to escaping into the head space. Taller columns will lengthen the time duration that the bubble is in contact with the water and can dissolve into the water. More importantly, taller columns will create a higher pressure at the bottom of the column. This high pressure will exert greater force on the surface of the bubble and force more gas into solution.Practical Application
Bubble diffusers can dissolve ozone into water efficiently; however, a fine pore diffuser must be used with a very tall water column. Water columns shorter than 10 feet typically achieve less than 50% mass transfer efficiency. Water columns 20 feet tall can achieve mass transfer efficiency up to 90%. This may not be practical in a given application. Fine pore diffusers can also plug with contaminates easier and cause poor long term performance. When designing a water treatment system using a bubble diffuser, keep safety in mind as high levels of undissolved ozone may escape from the head-space of the water.Venturi Injectors
A venturi injector is a very common method of ozone injection in industrial applications. A venturi injector combines a method for ozone injection, and provides good mass transfer efficiency in one device. A venturi injector requires a pressure differential across the device to create a vacuum to pull ozone gas into the device. Then, using mixing vanes the gas is thoroughly mixed with the water.A venturi injector creates very small bubbles desired for great mass transfer and a violent mixing action to dissolve gas into water. Using a venturi injector alone may achieve mass transfer rates of 90%.

Water Pressures
For a venturi injector to work properly there must be a pressure differential between the inlet and outlet of the device. This usually requires a separate water pump to increase the water pressure at the inlet of the venturi injector. It is then important that the outlet of the venturi injector is not obstructed or impeded in any way.We suggest placing pressure gauges directly at the inlet and outlet of the venturi injector. This will help with troubleshooting and determine the effectiveness of the device.
Off-Gas System
Using a venturi injector will require a method of removing the undissolved oxygen and ozone from the water. Unlike the bubble diffuser where the bubbles will naturally rise to the head-space and escape the piping system, a venturi injector has no method of removing this undissolved gas, one must be provided. A contact tank is a popular method, there are also de-gas chambers and columns that can be used. Ozone compatible air vents are used to remove this gas and vent to a safe location or to an ozone destruct unit.If an off-gas system is not used, the excess gas bubbles that may carry residual ozone can off-gas in undesirable locations causing safety concerns. Also, this excess gas may volatilize some of the dissolved ozone back into the gaseous form.
By-Pass and Plumbing
Venturi injectors become an integral part of the plumbing system in use. A pump is commonly placed prior to the injector, a tank after the injector. A by-pass loop is also commonly used to allow regulation of water flow through the injector and greater flexibility.Venturi Injector Performance and Sizing
Venturi injector sizing is a function of the water flow rate through the device. Water pressure will also play a factor in the determination of the venturi injector sizing. Each venturi injector is supplied with a performance chart illustrating the water flow, pressure and gas suction provided by that venturi injector.Water Back-Flow Prevention
When using a venturi injector it is necessary to use a device to ensure water cannot backflow from the venturi injector to the ozone generator. There are many devices used for this task: check valves, water traps and shut-off valves are all used. We have found the best success using a quality water trap in conjunction with a check valve to prevent all water back-flow.Diagram of system using a venturi injector, pump, contact tank and air vent.
Static Mixers
Static mixers are any static device designed for the sole purpose of mixing two flows together. In our application we are mixing ozone gas with water, therefore the same principle of breaking the bubbles up into the smallest possible bubbles is the goal with the static mixer.There are a variety of static mixers on the market, some go by trade names. For example Mazzei markets a static mixer under the name Flash Reactors. While there may be a variety of static mixers on the market they all serve the same function, dissolving ozone gas into water.
Sizing a Static Mixer
A static mixer is sized based on the velocity of water through the mixer. Each static mixer has vanes or mixing devices inside that require a specific velocity of water past those devices to achieve the desired results. This sizing will translate to water flow rate for our purposes. Each mixer should be sold and marketed with a range of flow rates that the mixer will work well with.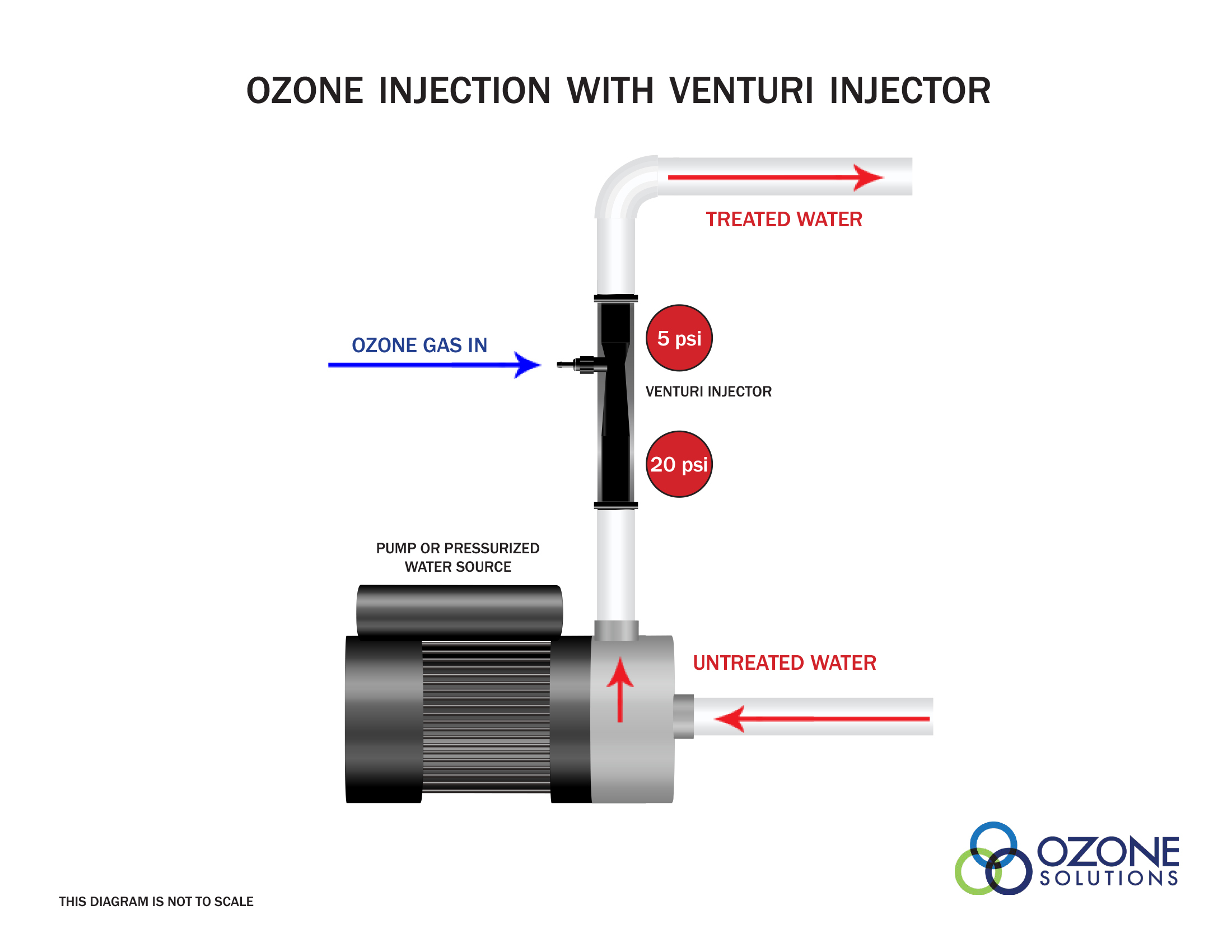
Ozone Injection
Ozone can be injected upstream of the static mixer using a tee or any other device to force ozone gas into the water stream. Then, the static mixer can be used to break up the gas into small fine bubbles to dissolve into water efficiently. Essentially a static mixer can be used in place of a venturi injector, this can be helpful when energy savings are desired due to the lack of necessary pressure differential.To force ozone gas into the water stream the ozone gas must be at a higher pressure than the water stream. Usually a pressure of 10 psi or greater is necessary to achieve gas flow into the water stream. This may eliminate the option of using only a static mixer and may require using a venturi injector to inject the ozone into water. The option of placing a static mixer in-line after the venturi is also an option.
Plumbing and Piping
A static mixer can be placed anywhere in a piping system intended to mix ozone gas with water. The best location when using a venturi injector to infuse ozone with the water is a few feet downstream of the injector. If using a contact tank or off-gassing column place the static mixer directly at the inlet of the tank with the venturi a few feet (as far as practical) upstream from the static mixer.Tips For Dissolving Ozone Into Water
Water Temperature
The solubility of ozone into water is temperature dependent. Lower water temperatures will achieve greater dissolved ozone levels due to a higher solubility rate. The solubility rate is the maximum ration of liquid to gas achievable for a given gas. While there are many other factors that will affect your mass transfer of ozone into water, it is very simple to understand that lower water temperatures increase solubility, if the solubility rate increases the mass transfer of ozone into water will increase.|
TEMPERATURE (°C)
*at atmospheric pressure |
SOLUBILITY |
|---|---|
| 0 | .64 |
| 5 | .5 |
| 10 | .39 |
| 15 | .31 |
| 20 | .24 |
| 25 | .24 |
| 30 | .15 |
| 35 | .12 |
Water Pressure
Water pressure will play a role in the solubility of ozone into water. When ozone gas is injected into water at higher pressures, more force will be placed on the wall of that gas bubble. This force will allow ozone to dissolve into water more efficiently. Any of the ozone injection methods will be more efficient when the entire system is operated at an elevated pressure. For example, water pressures of 35 psi will have about twice the solubility as water pressures of 10 psi.
Ozone Concentration
Ozone gas is normally measured in g/hr, however this is only a measurement of how much ozone is generated. Another method of measuring ozone is the concentration. More ozone in a given gas volume will mean that the gas has a higher concentration of ozone. This is normally measured in % by weight or g/m
3.
Ozone at higher concentrations will dissolve into water more efficiently than ozone at lower concentrations. See chart below for details.
Chart shows the saturation point of ozone in water based upon ozone concentration and temperature, at atmospheric pressure. Dissolved ozone level shown is mg/l
Water Quality
Any contaminate in the water that may affect water quality may also consume ozone, this will lower the dissolved ozone levels in the water. While this may be a desired effect due to the purpose of the ozone in water, it is important to take water quality into consideration when attempting to achieve a specific dissolved ozone level in the water.
A good example and often overlooked factor is chlorine in the water. Most city water supplies will have a chlorine residual in the water. When dissolving ozone into this water the ozone may react with the chlorine and consume some of the ozone.
Summary:
Dissolving ozone into water for any of the various applications listed above may be very simple or could be extremely complicated. This will be depending upon the application and the variables working within that application. This information should only serve to offer guidance on this process. For additional information refer to the great resources below or contact our office and speak with one of our Application Engineers.
